Evolve your EDC approach and empower your clinical research with ShareCRF.
Smart electronic data capture software that let storage and manage clinical research data from multiple sources in one place easily.

Smart electronic data capture software that let storage and manage clinical research data from multiple sources in one place easily.

Creating data capture systems that meet specific study needs is challenging, and ensuring their user-friendliness is even harder. ShareCRF streamlines this complex process, making clinical research data management simpler and more efficient.
Adapt ShareCRF to any study type, from decentralized to traditional, ensuring versatile performance for diverse clinical research needs.
Easily design clinical eCRF with our drag & drop editor. Without programming.
Collect patient data directly from their devices, and integrate it automatically in the EDC along with other clinical data.
Flexible, easy, integrated and with support for treatment stock management.
Code on-the-go using MedDRA and WHODrug: medical coding integrated with data.
Upload large files effortlessly, and with support for automatic anonymization to DICOM files.
Connects the EDC to other systems via our application programming interface.
In clinical trials, slow and unintuitive systems often lead to user frustration and operational inefficiencies, resulting in a cumbersome research process and jeopardized data quality. ShareCRF seamless addresses these challenges with its user-focused, intuitive, and streamlined design.

Overcome complex and repetitive challenges of clinical data management. ShareCRF’s cutting-edge automation handles the complexities, ensuring a smoother trial process.
Quickly set up your study with our intuitive drag & drop Visual Study Editor, no coding required. Reuse previous study designs, benefit from auto-generated documents (annotated CRF & blank CRF), and manage multiple CRF versions effortlessly, reducing time spent on data reconciliation.
Make mid-study changes easily with our EDC: use our secure test environment, to validate changes before publishing them, and when the changes are ready publish the new version (without having to pause the study) at the touch of a button. All this while maintaining data security and integrity.
Achieve high-quality data with our EDC: dynamic validation ensures real-time consistency, automated reminders boost user engagement and data accuracy, and our flexible monitoring system allows you to tailor data review workflows to the needs of each study.
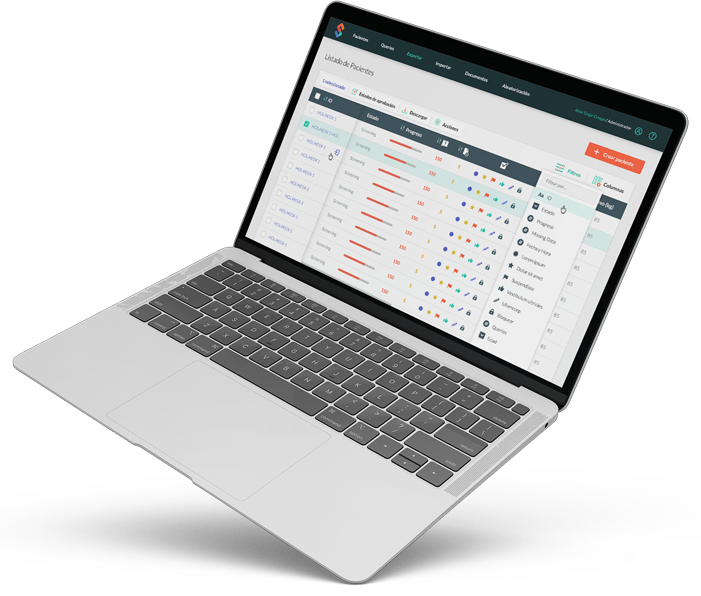
Improve control over your clinical trial with customizable notifications that allow you to stay informed of key events. Our reporting module provides intuitive visualizations of key metrics, allowing you to make data-driven decisions for better outcomes in your clinical trials.
In clinical research, swift and accurate support is crucial. ShareCRF stands apart with its in-house specialized technical team, providing precise, expert solutions right from the first interaction. No endless back-and-forth, just professional answers that keep your research moving forward.
Leverage ShareCRF to meet complex regulatory requirements in the simplest way. Designed to facilitate compliance with legal and regulatory standards. ShareCRF is validated using the GAMP5 methodology.























